Hair loss or alopecia is a common and distressing clinical complaint in the primary care setting and can arise from heterogeneous etiologies. In the pediatric population, hair loss often presents with patterns that are different from that of their adult counterparts. Given the psychosocial complications that may arise from pediatric alopecia, prompt diagnosis and management is particularly important. Common causes of alopecia in children and adolescents include alopecia areata, tinea capitis, androgenetic alopecia, traction alopecia, trichotillomania, hair cycle disturbances, and congenital alopecia conditions. Diagnostic tools for hair loss in children include a detailed history, physical examination with a focused evaluation of the child’s hair and scalp, fungal screens, hair pull and tug test, and if possible, light microscopy and/or trichoscopy. Management of alopecia requires a holistic approach including psychosocial support because treatments are only available for some hair loss conditions, and even the available treatments are not always effective. This review outlines the clinical presentations, presents a diagnostic algorithm, and discusses management of these various hair loss disorders.
Introduction
Hair loss in children encompasses a spectrum of conditions congenital and acquired, originating from hair shaft, follicular, or infectious causes. A congenital hair abnormality may be an isolated finding in an otherwise healthy child or a feature suggestive of a multisystem syndrome. Both congenital and acquired alopecia may be irreversible, resulting from destruction of hair follicles and replacement by fibrous scar tissue. Disease processes that lead to follicular loss are known as scarring alopecia, whereas follicles are generally preserved in non-scarring alopecia (1).
A basic understanding of hair biology enables consideration of normal versus abnormal hair loss in pediatric patients. Hair consists of the proteinaceous shaft and the root, anchored in the follicle, an involution of the epidermis. Hair follicles contain rapidly dividing cells, but the only visible portions are the follicular ostia, through which hair fibers emerge. Humans are born with a population of approximately five million preformed follicles. Follicles can produce lanugo, vellus, or terminal hairs. Newborns are covered in soft lanugo hair, which is replaced by downy, fine vellus hair after 3–4 months. At puberty, androgens transform most vellus hair into thicker, more pigmented terminal hair. Terminal hairs undergo cyclical growth with up to 90% of follicles in active growth (anagen phase); 1–3% in a brief involutionary transition (catagen phase); and 5–10% in dormancy (telogen phase). Hair is shed after 2–3 months in telogen phase, after which anagen begins again, and the cycle repeats. Daily loss of 50–150 telogen hairs is normal, but the number of hairs shed is roughly equal to the number of follicles entering anagen (2, 3).
Clinical presentation of pediatric alopecia ranges from subtle to disfiguring. A thorough history provides context as to whether hair was present at birth, or establishes acuity if hair was later lost. Other relevant details include diet and nutrition, environmental exposure to toxins, past medical history, and family history, with special attention to atopic, autoimmune, and endocrine diseases. Physical examination should focus on scalp health, followed by a survey of lashes, dentition, nails, and skin. Clinical findings, such as short stature, dysmorphic features, and vision or hearing impairment, could indicate an underlying syndrome.
Scalp examination reveals distribution of hair loss (diffuse, patterned, and focal) and the areas most affected (i.e., vertex, occiput, bitemporal, periphery). Trichoscopy is the application of a dermatoscope to directly visualize the scalp and hair, facilitating closer looks at regions of active disease. Trichoscopy is increasingly utilized in diagnosis of alopecias as trichoscopic features of common hair and scalp diseases are being recognized and organized (4–7). Notably, loss of follicular ostia identifies scarring alopecias, and preserved ostia indicate likely non-scarring alopecias. Trichoscopy also allows for identification of interfollicular stigmata, such as erythema, edema, pustules, and altered skin pigmentation or atrophy. As a non-invasive tool, trichoscopy is practical for use in children for diagnostic purposes and evaluating responses to therapeutic interventions (8). Hair quality can be evaluated with simple clinical maneuvers including the tug and pull tests. The tug test involves grasping hair between the thumb and forefinger near its root and tugging with the other hand on the same strand at its distal part and is positive if the hair fractures, indicating hair shaft fragility (9). To perform the hair pull test, gently pull a bundle of 20–60 hairs between the thumb and forefinger from multiple locations. The pull test is positive if greater than 10% of hairs are released; the test is uninfluenced by time since last hair wash (10, 11). In general, telogen hair comes out easily whereas anagen hair remains rooted in place. However, it is always important to evaluate roots of pulled hair under a microscope to definitively distinguish telogen from anagen hairs (Figure 1). Moreover, microscopy can exclude the presence of anagen hairs with thickened root sheaths, which would strongly suggest scarring alopecia even if the pull test does not reveal increased hair loss (12).
 Figure 1. (A) The anagen hair root is covered by a long sheath. (B) The telogen hair root is club shaped and without a sheath.
Figure 1. (A) The anagen hair root is covered by a long sheath. (B) The telogen hair root is club shaped and without a sheath.
The majority of pediatric alopecia is due to an acquired cause, most commonly tinea capitis, alopecia areata (AA), telogen effluvium, and traumatic hair loss, including trichotillomania and traction alopecia. If alopecia is present at birth and a congenital cause is suspected, aplasia cutis congenita (ACC) should be considered in the differential diagnosis. None of the above disease processes target and damage the follicle specifically; hence, all are non-scarring etiologies. However, tinea capitis and traumatic hair loss can be scarring from longstanding inflammation if left untreated. To aide the general pediatrician with distinguishing between various causes of alopecia, we include a diagnostic pathway that encompasses these and other congenital and acquired causes of hair loss in children (Figure 2; Table 1).
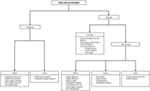 Figure 2. Approach to common etiologies of hair loss in children and adolescents.
Figure 2. Approach to common etiologies of hair loss in children and adolescents.
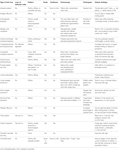 Table 1. Important clinical findings of alopecias to aid in diagnosis.
Table 1. Important clinical findings of alopecias to aid in diagnosis.
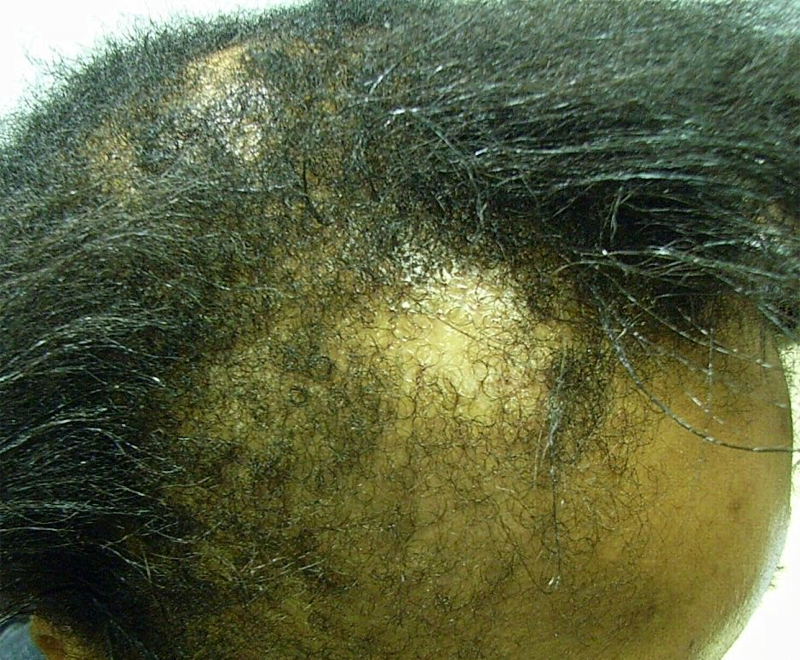
Tinea Capitis
Tinea capitis (Figure 3) is a common dermatophyte infection that primarily affects children, including infants. In the United States, Trichophyton tonsurans and Microsporum canis are the most common causative organisms. Trichophyton can spread human-to-human, whereas Microsporum can be acquired from pets (13, 14). After direct inoculation, the dermatophytes invade the hair follicle, resulting in acute patchy hair loss with various degrees of erythema, pruritus, and scaling (15). Kerions are boggy, sometimes purulent plaques that indicate presence of increased local inflammation (Figures 3B,C). Rare in infancy, kerions can present in children between ages 5 and 10 (16).
 Figure 3. (A) Patchy hair loss and broken hairs in a child with tinea capitis infection. (B) A boggy mass representing a kerion. (C) A large pustular kerion. (D) Black dots are remnants of broken hair, which have been removed on a gauze with gentle rubbing. (E) Close-up of broken hairs in culture tube.
Figure 3. (A) Patchy hair loss and broken hairs in a child with tinea capitis infection. (B) A boggy mass representing a kerion. (C) A large pustular kerion. (D) Black dots are remnants of broken hair, which have been removed on a gauze with gentle rubbing. (E) Close-up of broken hairs in culture tube.
Differential diagnosis of tinea capitis includes AA and ACC. The presence of black dot hairs (broken hairs within the lesion), significant erythema and scaling, and posterior cervical lymphadenopathy all favor tinea capitis. Both a swab culture of the affected skin and a hair from the affected area should be submitted for fungal culture (Figures 3D,E). Fungal potassium hydroxide preparations rapidly identify dermatophytes, and dermatophyte culture will confirm the diagnosis. Notably, sampling kerions can yield false-negative cultures because kerions mostly reflect an intense inflammatory response (17). Patients can be successfully treated with oral antifungal medications (e.g., terbinafine and griseofulvin) plus antifungal shampoos such as ketoconazole 2% shampoo to prevent shedding. Many children under 1 year can be adequately treated with topical azoles alone. Kerions can be managed with gentle soaks or keratolytic emollients (18, 19). Since dermatophyte infections can be spread to close contacts, sharing of combs, hairbrushes, and hats should be avoided. In some cases, treatment of close contacts might include preventative use of ketoconazole 2% shampoo for hair washing.
Alopecia Areata
Alopecia areata is a common form of autoimmune hair loss with a lifetime prevalence of approximately 2%, occurring at any age (20). Approximately 20% of patients are under 16 and 1–2% present before age 2 (21, 22). The lifetime risk for AA is increased in individuals with personal or family history of other autoimmune disorders such as vitiligo or thyroid disease (23). The genetic basis for AA is still under investigation; however, genes that control T-cell proliferation and activation, including CTLA4, interleukins (IL2/IL21), human leukocyte antigens, and natural killer cell-activating ligands, have been implicated in AA pathogenesis (24).
The clinical course of AA is typified by patchy hair loss on the scalp (Figure 4A), though some individuals progress to have hair loss of the entire scalp [alopecia totalis (AT)] or the entire body [alopecia universalis (AU)]. It has been estimated that approximately 5% of patients with AA will develop AT and 1% will go on to develop AU. Although patches of hair loss can develop randomly over the scalp, there are patterns of hair loss that can occur as well, such as the band-like pattern of hair loss on the lower occiput scalp from ear to ear (ophiasis type), or as vertex hair loss with occipital and temporal sparing (sisaipho type) (25). Clinical diagnosis of early disease can often be made based on observing non-scarring, well-demarcated areas of complete hair loss, exclamation mark hairs observed at the lesion peripheries (Figure 4B), and positive hair pull with microscopic evidence of tapered hair (Figure 4C). Nail changes, including trachyonychia (rough vertically arranged linear ridges in the nail plate) and pitting (pinpoint dotted depressions in the nail plate), may be associated with AA. Clinical features that suggest a more chronic, relapsing course include a family history of AA, evidence of nail changes, presentation of areata during childhood, duration of current hair loss episode greater than 1 year, patient history of atopy, and more severe involvement (AT or AU). Though rarely necessary, scalp biopsies demonstrate the histological hallmark of dense lymphocyte infiltration of anagen hair bulbs and dermal papillae.
 Figure 4. (A) Single, well-defined patch of hair loss characteristic of alopecia areata (AA). (B) Pathognomonic exclamation point hairs in AA. (C) Light microscopic appearance of a tapered hair removed from the scalp of patient with rapidly progressive AA.
Figure 4. (A) Single, well-defined patch of hair loss characteristic of alopecia areata (AA). (B) Pathognomonic exclamation point hairs in AA. (C) Light microscopic appearance of a tapered hair removed from the scalp of patient with rapidly progressive AA.
No consistently effective treatment exists for AA, with even fewer therapeutic options for children. Patients with a few patches of alopecia can be managed by watchful waiting as spontaneous regrowth can occur within 6–12 months in up to 50%. Children under 10 years often respond well to topical steroids (15). The side effect profile is considered benign, although development of rare systemic effect must be monitored. As hair regrows, topical steroids should be cautiously continued while watching for dermal atrophy. For children over 10 years with limited areas of hair loss, intralesional steroid injections may be trialed as tolerated. In our experience, treatments can be performed rather painlessly with the use of a topical anesthetic and vibrational device during injections. Systemic corticosteroids are reserved for AA refractory to other local treatment (26). Non-steroidal treatments include phototherapy, topical minoxidil, topical immunotherapy, such as squaric acid dibutyl ester and diphencyprone, and immunomodulators. Squaric acid dibutyl ester can increase hair growth particularly in children with extensive involvement, although side effects include itchy dermatitis, blisters, cervical lymphadenopathy, and hyperpigmentation. Children with AT or AU are often poor responders (27, 28). Diphencyprone has some efficacy in children with extensive involvement and the regrowth may be maintained in some patients even after discontinuation of diphencyprone (29). Systemic treatment with oral tofacitinib, a Janus kinase 1/3 inhibitor, has recently been reported as a successful treatment in pediatric patients with extensive AA. In a study of 13 patients’ ages 12–17 years, 9 patients (70%) had clinically significant hair regrowth after approximately 6 months of treatment. Although long-term side effects including potential for malignancy are unknown and require discussion with patient and family, overall initial adverse events appear to be mild and largely limited to upper respiratory infections, headache, and transient mild elevations in liver transaminase levels that returned to normal despite continued tofacitinib treatment (30). However, AA in its widespread forms remains hardest to treat, and no treatment appears to prevent relapse.
Hair Cycle Disturbances
Telogen Effluvium
Telogen effluvium is a common non-scarring hair cycle disorder in which an abnormal amount of hairs transitions from anagen phase to telogen phase, resulting in increased diffuse shedding of hair (19, 31). This growth cycle perturbation is usually due to medications, illness, surgery, metabolic disturbances, nutritional deficiencies, trauma, immunizations, and the postpartum state. However, no trigger is identified in one-third of cases (19, 31–34). Moreover, it is not uncommon for telogen effluvium to be superimposed on other forms of alopecia if patients experience an inciting event. Important differential diagnoses to consider include androgenetic alopecia (AGA), female pattern hair loss (FPHL), and diffuse AA (35, 36). Acute telogen effluvium occurs 3–4 months after an inciting event, lasts approximately 3 months, and spontaneously resolves, while chronic telogen effluvium usually lasts longer than 6 months. The hair pull test roughly estimates the percentage of telogen hairs. A positive hair pull test, particularly at the vertex and the scalp margins, is highly suggestive of telogen effluvium (19, 31, 33).
Patients can present after the phase of acute shedding has subsided. In these cases, patients report a period of increased shedding, while on exam, clinicians observe evidence of short hairs regrowing along the temporal and frontal hairline. A complete blood count and metabolic panel, with additional tests for metal poisoning and nutrition deficiencies, can help identify causes of telogen effluvium (19, 37). Acute telogen effluvium is completely reversible with removal of stressors and treatment of underlying disorders, if present. Prognosis is generally good with most patients regrowing hair within the year, though at a slow rate of approximately 1 cm/month (19). Treatment is not necessary except in cases of chronic telogen effluvium, but unfortunately, options are limited (38, 39). While topical minoxidil has mixed results in treating telogen effluvium in adults, its efficacy in children remains unknown (39).
Anagen Effluvium
Anagen effluvium is rapid hair loss that is rarely seen in the healthy pediatric population. It usually occurs within days or weeks after an inciting event resulting in profound alopecia affecting 90% of the scalp, corresponding to the number of hairs typically in the anagen phase (31, 40). Anagen effluvium should be suspected in a patient with a history of radiotherapy; antineoplastic agents (doxorubicin or vincristine) and other medications, including colchicine; toxic chemical exposure (mercury, boron, and thallium); and severe malnutrition (31, 40, 41). These insults acutely disrupt mitotic activity, thus the proliferating cells of the hair bulbs are preferentially targeted, while quiescent stem cells within the follicle remain unaffected (41). Thus, breakages occur at the fragile proximal hair shaft. Dystrophic anagen hairs will often have tapered fractures of hair shafts (31, 41). Once the insult is removed, most patients experience normal hair regrowth with a delay of 1–3 months; however, permanent alopecia may occur depending on the underlying cause (41). It is important to note that pediatric patients treated with myeloablative conditioning with busulfan have been reported to have permanent diffuse alopecia as a potential side effect. In one multicenter study of 240 childhood leukemia patients, 66 received busulfan and 174 received total body irradiation (TBI) as conditioning. In the busulfan group, 25.8% reported alopecia as a long-term side effect of treatment >10 years out from their stem cell transplant, whereas only 2.9% reported this in the TBI-treated group (42). Use of scalp hypothermia (scalp temperature <24°C), scalp compression, and topical minoxidil can reduce hair loss in some adult patients undergoing chemotherapy, but the techniques differed widely in these studies, and the benefit in children remains unclear (41, 43, 44).
Loose Anagen Syndrome (LAS)
Loose anagen syndrome is a relatively infrequent cause of hair loss, characterized by poor anchoring of anagen hairs to the follicle, resulting in frequent painless hair loss with gentle pulling (31, 45). Patients are born with sparse but normal hair, and generally present between 2 and 7 years of age with unruly hair that is slow growing. Light microscopy and trichogram reveal predominantly loose anagen hairs (>50%), misshapen anagen roots with absent external root sheaths, and ruffled cuticles (46). Trichoscopy findings include rectangular black granular structures, solitary yellow dots, and >90% of follicular units with single hairs (47). LAS is considered autosomal dominant with incomplete penetrance, and a keratin mutation is responsible for several familial cases (46, 48, 49). LAS may also be associated with heritable developmental conditions, such as Noonan syndrome (50, 51). Due to abnormal premature keratinization in the inner root sheath, hair and the inner root sheath are poorly adherent in LAS. Most cases require no treatment as hair spontaneously returns to normal within a few years, but avoiding hair products and styling, and gentle handling may prevent further hair loss (31, 48, 51). Topical minoxidil may benefit LAS patients, particularly those with severe symptoms (46, 49).
Short Anagen Syndrome (SAS)
Short anagen syndrome is a rare cause of perceived hair loss in children, due to a shortened growth phase of the hair cycle. This is typically a sporadic congenital condition that can be inherited in an autosomal dominant fashion in some cases. Patients present with normal hair density but with very short hairs that never need to be cut, similar to LAS. However, a gentle hair pull in patients with SAS will reveal that the short hairs are shed in the telogen phase in contrast to being shed in the anagen phase in LAS. This condition is benign, and the majority of patients will improve by puberty (52, 53).
Traumatic Hair Loss
Trichotillomania
Trichotillomania is a not infrequently seen impulse control disorder characterized by repetitive, compulsive, and self-induced hair pulling from the scalp, eyebrows, and/or other parts of the body (19, 31). Although trichotillomania can occur in children under 6 years old, peak incidence is age 9–13 years with a strong female predominance (54, 55). Clinical presentation includes multiple non-scarring and focal patches of hair loss with irregular borders (Figure 5), little to no scale or excoriation, and presence of broken hairs of different lengths, often contralateral to the side of the body with the dominant hand (19, 56). Hair loss in trichotillomania frequently spares the occiput, as these hairs are more painful to pull out. Trichobezoars can be identified in patients who eat their pulled hairs, leading to gastrointestinal symptoms, including nausea and vomiting, abdominal pain, and bowel perforation (57). In trichotillomania, hair density is normal with a negative pull test; however, presence of flame hairs (proximal hair residue that remains after pulling anagen hairs), the “v-sign” (two hairs rising from the same follicular opening and broken at same level), and tulip hairs (short hairs with darker tulip-shaped ends) on trichoscopy are highly characteristic (58, 59). The main differential diagnosis of trichotillomania is AA. Distinguishing features that are more suggestive of trichotillomania include sparing of the lower occiput scalp, no discrete patches of complete hair loss, a geometric-shaped patch with hairs of all different lengths sometimes with associated evidence of scalp trauma (small pinpoint hemorrhagic spots on scalp). This is in contrast to a patch of hair loss that spontaneously regrows in patients with AA where the hairs typically are all the same length.
 Figure 5. Patchy hair loss characterized by irregular borders and hairs in various stages of regrowth in a child with trichotillomania.
Figure 5. Patchy hair loss characterized by irregular borders and hairs in various stages of regrowth in a child with trichotillomania.
Trichotillomania is classified under obsessive–compulsive and related disorders in DSM-V. While its causes are unknown, higher rates of obsessive–compulsive disorder and psychiatric conditions are found in first-degree relatives (56, 60, 61). Management varies across age of presentation. Trichotillomania in preschool-aged children is considered a habit disorder similar to thumb sucking and has a benign course generally disappearing by school age. This can be managed conservatively with gentle reminders. In preadolescents and teenagers, trichotillomania often presents with other psychiatric comorbidities, and patients may benefit from a psychiatric evaluation if willing (19, 55, 56). In children and adolescents, behavioral therapy—positive reinforcement, habit reversal training, and self-monitoring—are effective when working with a skilled therapist (62, 63). Pharmacological interventions are limited. Studies showed mixed results for selective serotonin reuptake inhibitors and tricyclic antidepressants in adults, and data regarding their efficacy in children are lacking (55). While N-acetylcysteine is a promising agent that reduces symptoms in adults, no benefit was seen in children (64, 65).
Traction Alopecia
Traction alopecia is a common pattern of traumatic hair loss caused by constant tensile forces exerted on scalp hair. Clinical presentation follows a predictable natural history, with early development of perifollicular erythema, pustules, scaling, and broken hairs and late sequelae of follicular scarring and permanent alopecia. In children, marginal hair loss along the temporal hairline is commonly observed, although non-marginal occipital and frontomarginal patterns can also occur depending on hairstyle (66). Fringe sign is the retention of hairs along the frontal or temporal rim (Figure 6A). Highly suggestive of excessive tension, tenting of hair follicles refers to the raising of skin on the scalp when hair is pulled extremely tightly (Figure 6B). Both are important exam findings for diagnosing traction alopecia (67, 68). The applied trauma is unintentional and often secondary to cultural and social practices given known associations with grooming accessories and particular hairstyles (68, 69). High-risk hairstyling practices include tights buns, ponytails, braids, cornrows, or dreadlocks; application of weaves, braids, or hair extensions to relaxed hair; and any hairstyles that cause pain, crusting, tenting, or pimples (68). Treatment involves adopting grooming practices that minimize traction, and antibiotics to address folliculitis if present (31). With early diagnosis and treatment, regrowth can occur within months; however, chronic traction alopecia may be irreversible despite discontinuation of traction.
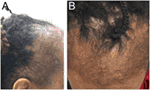 Figure 6. Fringe sign (A) and tenting (B) are clinical findings suggestive of traction alopecia.
Figure 6. Fringe sign (A) and tenting (B) are clinical findings suggestive of traction alopecia.
Central Centrifugal Cicatricial Alopecia (CCCA)
Central centrifugal cicatricial alopecia is a form of scarring alopecia that starts with patchy hair loss at the vertex and gradually spreads centrifugally. Patients may complain of tender or pruritic bumps in this location of the scalp. Long thought to be secondary to damage from chemical relaxers and hair dyes, CCCA disproportionately affects middle-aged women of African descent and was previously rarely reported in children (70, 71). However, a recent pediatric case series suggests a familial mode of inheritance with earlier disease onset and greater penetrance in individuals of successive generations. A case series of six children with CCCA reported that the majority (83%) were of African descent, had no history of chemical treatment of hair, and had a positive family history of CCCA (72, 73). Although still a rare cause of hair loss in children, a low threshold of suspicion should be maintained for young African American patients presenting with vertex hair loss and symptoms. To the best of our knowledge, CCCA has not been reported in children of non-African descent. Early treatment with topical or intralesional steroids can decrease inflammation to slow or halt disease progression. This highlights the importance of recognizing CCCA in children with a positive family history to control progressive scarring alopecia.
Discoid Lupus Erythematosus (DLE)
Discoid lupus erythematosus is very rare in children. It is an inflammatory autoimmune disease that most commonly leads to erythematous, scaly patches on the head and neck that can spread to involve other areas of skin in some cases. The inflammation can lead to atrophy and scarring of the skin and permanent hair loss. Lesions may initially mimic tinea capitis or bacterial infections given the confluent erythema, but laboratory investigations will be negative and there will be a lack of response to anti-infectious agents. The diagnosis is typically made by scalp biopsy revealing basal vacuolization, increased dermal mucin, and a dermal, perivascular, and periadnexal lymphocytic infiltrate. Although there is a female predominance in adult DLE, there is no sex predilection in children. Antinuclear antibodies are uncommon in children (26%), as opposed to 43% positivity in adults; however, progression to systemic lupus erythematosus (SLE) is more common in children with 26% over a 3-year period developing SLE in contrast to only 5% of adults (74). One retrospective study of 40 children with DLE reported the greatest risk of progression to SLE was in the first year after DLE diagnosis. The majority of patients met SLE diagnostic criteria with mucocutaneous disease, cytopenia, and positive antibodies (75). Therefore, children with DLE should be very closely monitored for SLE development. Other goals of management involve controlling activity of disease to prevent further scarring and dyspigmentation. Treatments include potent topical steroids, hydroxychloroquine (4–6 mg/kg/day), and intralesional or systemic corticosteroids. There appears to be some genetic predilection, with DLE patients often reporting a family history of rheumatologic conditions.
Lichen Planopilaris (LPP)
Lichen planopilaris is an exceedingly rare cause of scarring alopecia in the pediatric population. The cause of LPP is unknown and seems to be sporadic. LPP in adults typically occurs in Caucasian, postmenopausal females. In contrast, LPP in children has no sex predilection and has been reported to occur more frequently in patients of East Indian and African descent. Patients present with scarring alopecia with perifollicular erythema and scales on exam, in contrast to the more confluent erythema and scaling seen in DLE. About half of LPP cases are asymptomatic and could be confused with the more common AA as a result. The clinical features of atrophy and perifollicular scale and erythema are strongly suggestive of LPP (76).
Aplasia Cutis Congenita (ACC)
Aplasia Cutis Congenita (ACC) is a relatively infrequent heterogenous group of diseases characterized by focal absence of skin with accompanying scarring alopecia at birth. Clinical subtypes are characterized by location and pattern of skin absence, presence of associated malformations (e.g., trisomy 13 or 4p-syndrome, cleft lip and palate), and mode of inheritance (77). Although truncal and limb involvement has been described, most patients present with a single midline scalp lesion (Figure 7) (78). Typical lesions are well circumscribed and may be ulcerated or covered by a tense epidermal membrane. However, unlike other neonatal blistering disorders, ACC involves both epidermis and dermis. Proposed etiologies for ACC include infection, vascular malformations, and teratogens affecting aminogenesis or ectodermal fusion, but no unifying theory has been identified (77, 79, 80). Most lesions spontaneously reepithelialize over months, leaving behind a hypertrophic or atrophic scar. Thus, supportive wound care is recommended unless the lesion is greater than 3 cm, in which case split-thickness skin grafting may be indicated (81).
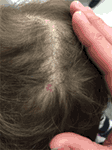 Figure 7. A single, subcentimeter aplasia cutis congenita lesion on the vertex of the scalp.
Figure 7. A single, subcentimeter aplasia cutis congenita lesion on the vertex of the scalp.
Other Congenital Alopecia
Transient Neonatal Hair Loss (TNHL)
Transient neonatal hair loss encompasses the very common phenomenon of neonatal occipital alopecia and development of bald patches in other locations. Healthy babies have been observed to develop alopecic oval patches, most notably in the occiput, during the first 2 months of life. Friction was the presumed cause of TNHL, with the occiput particularly affected due to supine sleeping positions. However, recent retrospective studies found no association between TNHL and sleeping positions. TNHL has a benign course with no accompanying symptoms, and the hair spontaneously regrows (82, 83).
Congenital Triangular Alopecia (CTA)
Congenital triangular alopecia, also known as temporal triangular alopecia or Brauer nevus, is a non-progressive, non-scarring infrequent cause of alopecia that primarily affects frontotemporal regions. Lesions are triangular or lancet shaped, with complete hair loss of the affected patch except a few vellus hairs. Most lesions are unilateral, predominantly left-sided, but can be bilateral in approximately 7.5–20% of cases (84, 85). A small subset of CTA is associated with known syndromes, including Down syndrome and phakomatosis pigmentovascularis. Although CTA can manifest at birth, most cases are reported after age 2 when the affected patch becomes more noticeable as children undergo vellus to terminal hair transition. Vellus hairs are a highly sensitive but not specific marker for CTA (86). Trichoscopy can demonstrate other features such as miniaturized terminal hair follicles, hair length diversity, and the absence of features characteristic for other types of alopecia, notably AA (85). CTA remains unchanged throughout adult life. Surgical intervention can produce satisfactory cosmetic results, and efficacy of topical minoxidil has been described in a 1-year-old patient (87–90).
Congenital Atrichia and Hypotrichosis
Congenital atrichia and hypotrichosis comprise a group of rare congenital disorders that present with hair loss or reduction in hair volume (31). Caused by autosomal recessive mutations in the human hairless gene, atrichia congenita presents with either complete hair loss shortly after birth or permanent absence of scalp hair due to follicular agenesis or programmed follicular destruction and papular lesions on the face, scalp, and extremities (31, 91–93). Vitamin-D-dependent rickets is an important differential diagnosis to exclude (93, 94). Atrichia congenita is often mistaken for AU until patients fail to respond to steroid therapy (95). Congenital hypotrichosis is less severe than atrichia congenita with patients presenting after 2 years with diffusely thin hair and variation in quality and quantity of hair (31). Congenital hypotrichosis may be associated with other features, including epilepsy, chromosomal abnormalities, inborn errors of metabolism, and ocular, ectodermal, or skeletal abnormalities. If any associations are identified, workup for a syndromic cause should be performed and genetic counseling may be appropriate depending on the diagnosis (96). For many of these conditions, there is currently no effective treatment.
Other Patterned Alopecia
Androgenetic Alopecia (AGA)
Androgenetic Alopecia (AGA) is characterized by relatively common non-scarring hair loss in androgen-dependent regions of the scalp, particularly the vertex and temporoparietal areas, with distinct and well-described male patterns (31, 97, 98). The most frequent cause of alopecia in adolescents, AGA presents gradually after puberty when enough testosterone is converted into dihydrotestosterone and is not generally observed in prepubertal patients without abnormal androgen levels (31, 99, 100). Trichoscopy reveals increased number of thin and vellus hairs, hair shaft thickness diversity, perifollicular pigmentation, and variable number of yellow dots. Biopsy commonly demonstrates perifollicular inflammation (100, 101). A strong genetic predisposition is observed, but no causative gene has been identified, although the androgen receptor gene has been posited (102, 103). Topical minoxidil can prevent progression of AGA in adolescents (99, 100). Finasteride with or without minoxidil is equally well-tolerated and increased hair density in a small cohort of adolescent boys (100). Recent studies have also demonstrated efficacy of low-level laser therapy for adult patients of both genders with AGA (104–106).
Female Pattern Hair Loss (FPHL)
Female Pattern Hair Loss (FPHL) is a commonly seen non-scarring alopecia distinct from male AGA, presenting with diffuse central thinning beginning anytime after menarche or adrenarche (100, 107). Frontotemporal recession or vertex loss, a more “male pattern” baldness, is less common (107). Trichoscopy shows increased yellow dots, pilosebaceous units with only one hair, and perifollicular hyperpigmentation (108). Family histories for FPHL are often not straightforward, and androgens may not explain all cases of FPHL as many female patients demonstrate little to no biochemical or clinical evidence of increased androgens (107, 109). Signs of hyperandrogenism such as hirsutism and menstrual disturbances should prompt evaluation for polycystic ovarian syndrome, congenital adrenal hyperplasia, and other androgen-producing conditions (110, 111). Topical minoxidil is similarly well tolerated in adolescent girls and prevents progression of FPHL (100). In cases of hyperandrogenism, antiandrogen or 5-alpha reductase inhibitors, such as spironolactone, finasteride, and cyproterone acetate, can be used, though the efficacy of these treatments in children remains unclear (107, 112).
Hair Shaft Disorders
Hair shaft disorders are acquired or congenital conditions characterized by defects in the hair structure with or without increased breakage. Patients often present with an inability for appropriate hair elongation, abnormal hair texture, or abnormal hair appearance. To help distinguish between the hair shaft disorders, a tug test can be performed to assess for hair fragility (113). Moreover, light microscopy and trichoscopy can identify features specific to different hair shaft disorders (31, 114). Acquired hair shaft disorders such as trichorrhexis nodosa or bubble hair are common and can occur after damage from hair styling, chemicals from hair dyes or permanents, or mechanical trauma from brushing (113). Avoidance of these practices that can be traumatic to the hair shaft will often lead to improvement.
In contrast, congenital hair shaft disorders are very rare and have been associated with many genetic factors, including keratin genes (monilethrix), a transcription factor involved in nucleotide excision repair (trichothiodystrophy), a serine protease inhibitor (trichorrhexis invaginata), genes involved in the urea cycle (trichorrhexis nodosa), and plakoglobin and desmoplakin genes (pili torti, or Woolly hair syndrome) (31, 115). No effective treatments are available for most congenital hair shaft disorders. Some congenital hair shaft disorders such as pili torti may improve over time, while oral retinoids and topical minoxidil improves clinical symptoms of monilethrix (116–121). A comprehensive, algorithmic approach to diagnosing hair shaft disorders has been previously written to address these much less common causes for hair loss and may be used if a hair shaft disorder is suspected (113).
Conclusion
Alopecia is a relatively frequent presenting problem in the pediatrician’s office and can be due to diverse congenital or acquired conditions. Assessment based on the patient’s age, acute or chronic timeline, and scalp examination can provide extensive clinical insight into the etiology of alopecia. Often a referral to a pediatric dermatologist is helpful, as trichoscopy, light microscopy, and biopsy may not be available in the pediatrician’s office. Physicians can offer psychosocial support with all hair loss complaints. Some alopecia conditions respond to therapeutic interventions, while others resolve over time, especially after removal of the insult. Still others remain permanent without effective therapies. Nonetheless, timely identification of the hair disorders will lead to effective counseling of patients and families about the specific etiologies, prognoses, and available treatment options.
[“source=frontiersin”]