Abstract
Autologous keratinocyte cultures and combinations of scaffolds, different cell types, solutions of macromolecules, or growth factors have contributed to the resurfacing of burns and large wounds. There are, however, significant limitations with these therapies. No tissue-engineered substitute can fully replace the split-thickness skin graft for permanent coverage of full-thickness skin loss in one step, and none contain a functional vascular plexus. Current research characterizes skin as more than a barrier with sensory function, but as an intricate biological factory participating in cell signaling, metabolism, and protein synthesis, and as a vital component of the nervous, immune and endocrine systems. This paper provides a comprehensive review of the structure and function of skin, highlighting the importance of regenerating an organ that will function physiologically.
1. Introduction
Skin has several functions. It provides a barrier to water loss and pathogens and protects against diverse forms of trauma, including thermal, chemical and ultraviolet radiation. Skin keeps us in touch with our environment through a host of nerve endings, regulates body temperature and enhances metabolic functions, as well as synthesizing vitamin D [1]. To fulfill this purpose, regenerated skin requires three integrated structural characteristics: 1. a layered interface, 2. epidermal appendages, and 3. mechanical stability.
2. Layered interface
Many organs have a series of layered interfaces (Fig. 1A); an avascular cellular epithelium that spontaneously regenerates, a basement membrane zone (BMZ) and stroma or vascular supporting connective tissue that does not regenerate [2]. In skin (Fig. 1B), these layers are referred to as an epidermis of stratified squamous epithelium, a BMZ and a fibrous neurovascular dermis which rests on a hypodermis or subcutaneous fat.

The epidermis is mainly composed of sheets of keratinocytes (Fig. 2) but also contains non-epithelial cells, including antigen-presenting dendritic Langerhans cells as well as melanocytes and Merkel cells. The epidermis is nourished by diffusion of intercellular fluids, from the dermal vasculature [3].

There are three distinct layers of nucleated cells. Approximately every 28 days, fully differentiated cuboidal basal keratinocytes with large nuclei, abundant organelles, and a phospholipid membrane migrate apically from the basal layer through the spinous and granular layers [4]. During this turnover process, an accumulation of keratin and lipids ensues which then undergoes terminal differentiation to form the stratum corneum.
Among their functions, keratinocytes proliferate to heal wounds, transport water and urea through aquaporins, receive melanin from melanocytes, control water permeability and participate in innate and adaptive immunity through antimicrobial peptide secretion and through the presence of Langerhans cells, respectively [5].
Of note, keratinocytes do not exist in isolation. Signaling pathways for growth and differentiation of skin through mitotic spindle orientation require molecular interactions between various cells [6]. The whole group is referred to as an interactome and gives the cells polarity, ensuring correct cellular orientation [7].
Reinforcing the epidermis is the dermis, that accommodates the vascular, neural, lymphatic and adnexa of the skin. The dermis provides a durable base that can absorb mechanical forces to prevent shear. It is described as having a superficial papillary zone, comprising relatively thin collagen fibers (Fig. 2), and a much thicker reticular dermis – a compact layer of thicker collagen fibers [8]. The primary cell type is the fibroblast, which produces the extracellular structural proteins, glycosaminoglycans, collagen and elastin fibers (Fig. 2), the latter enhancing the deformability of the dermis. Between the cells and fibers is the extracellular matrix, composed mainly of glycosaminoglycan/ proteoglycan molecules, which hydrate the tissue due to the high water binding capacity of hyaluronic acid [9].
3. Epidermal appendages
Hair follicles, sebaceous glands and sweat glands undergo constant cellular turnover to replace senescent cells and those exfoliated from the surface of the skin.
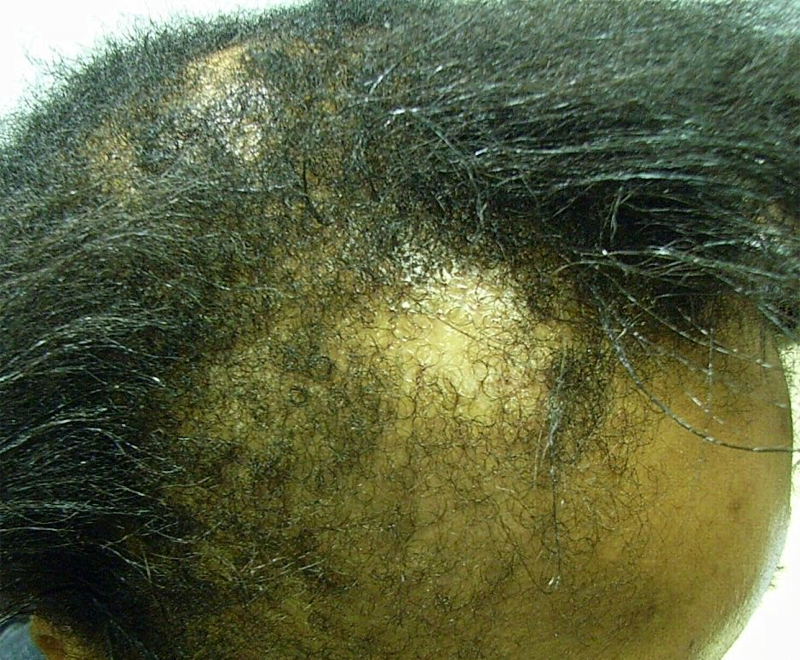
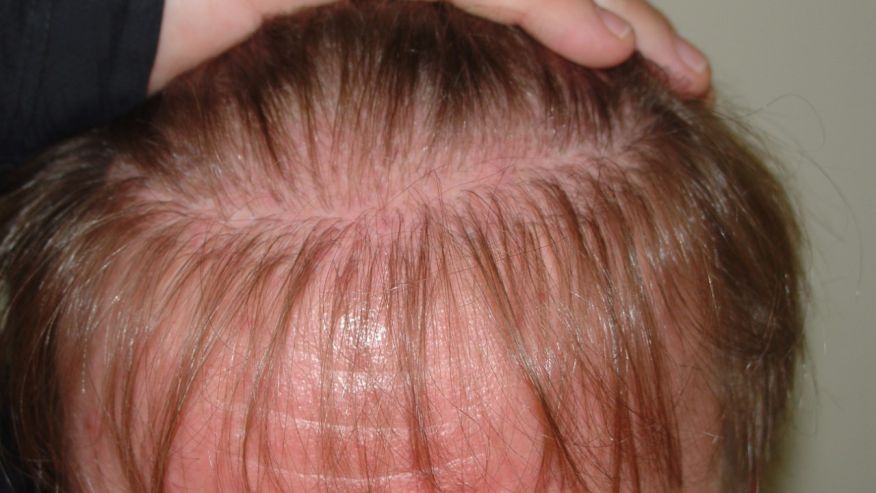
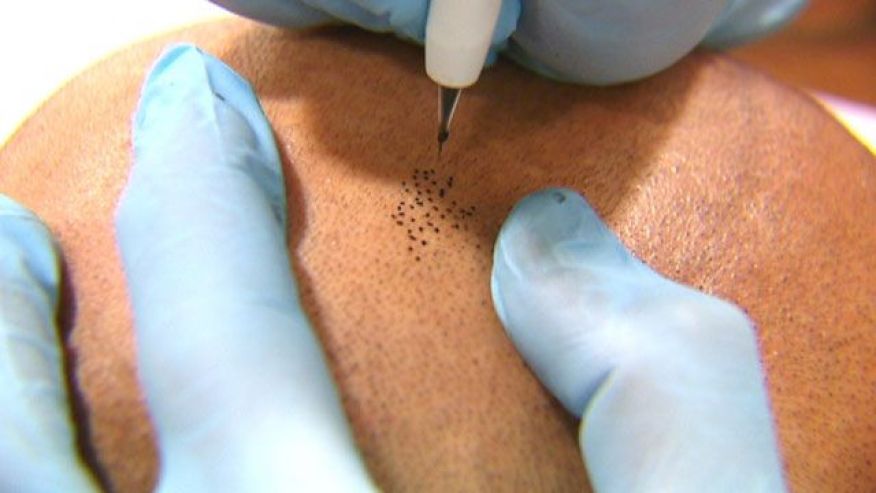
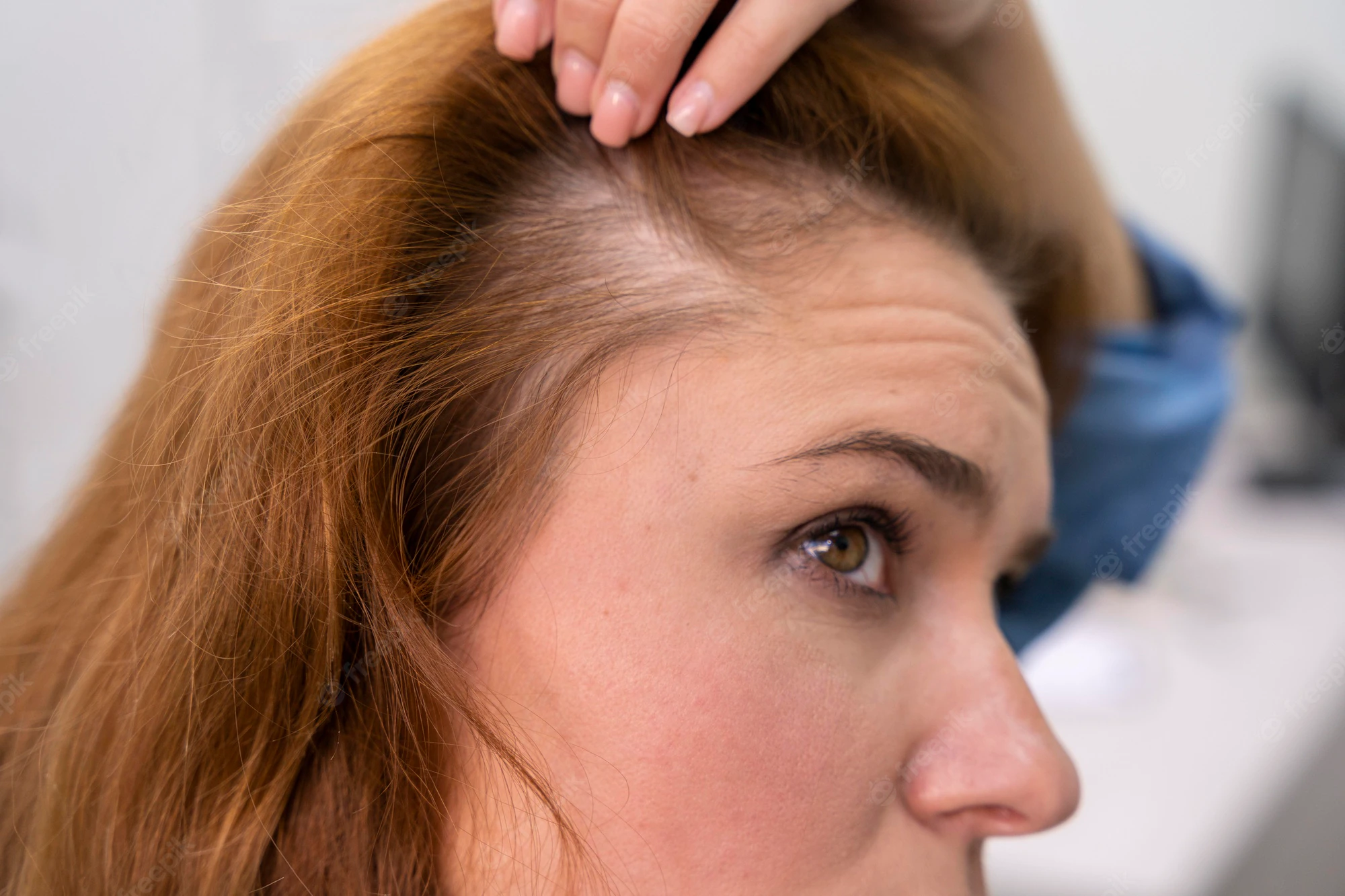
Efforts to regenerate skin from hair follicles was attempted as early as 1875 when Ernst Schweninger, Assistant Professor at the Institute of pathology in Munich, “grafted” hairs pulled out with their roots and placed these onto granulating wounds [10]. He observed that within 3–5 days epithelial islands formed around the hair roots. His investigations might have received more attention with the advantage of modern imaging technology (Fig. 3).

The hair shaft is about one-tenth of a millimeter wide and weighs only a few millionths of a gram. This organized and segmented structure originates from a major stem cell reservoir, in the outer root sheath below the sebaceous gland, called the follicular bulge [11]. Here the 7-transmembrane receptor, leucine-rich G-protein coupled receptor 5 (Lgr5) is a marker of the Wnt-regulated adult stem cell population. Expression mapping has also found Lgr6+ epithelial stem cells, a close homolog of Lgr5, in a Wnt-independent niche in adult skin just above the bulge, in the isthmus region of the hair follicle [12]. Under physiological conditions, these follicular stem cells and their progeny contribute only to regeneration of the hair follicle, the isthmus and the sebaceous gland [[13], [14], [15]]. The interfollicular epidermis (IFE) is maintained by stem cells in the basal layer of the epidermis that give rise to short-lived progenitors that produce epidermal proliferative units, while sebaceous-gland cells are maintained by progenitors located above the bulge, which express the Blimp1 protein [16].
When the skin is injured, however, the follicular cells are actively recruited to produce all the cell lineages of skin including the IFE – a process requiring complex epithelial-mesenchymal interactions [12,17].
The development of epithelial culture systems facilitating the growth of human skin from isolated Lgr6+ adult stem cells has led to a breakthrough in the resurfacing of full-thickness skin defects (Fig. 4) [18].

4. Mechanical stability
Thirdly skin must be mechanically stable. Prevention of shearing of the epidermis from the dermis is paramount. This is favored by several mechanisms. Neighboring keratinocytes, bound to each other by desmosomes, and a meshwork of tonofilaments provide a degree of epidermal stability. The epidermis is bound to the dermis by the interlocking of its epidermal projections into the dermis (rete pegs) and the complementary dermal capillary loops (papillae) projecting into the epidermis, a mechanism that increases the surface area for binding interactions between these layers [19].
More important, however, is the BMZ, a complex continuum of macromolecules which form a network providing a strengthened connection of the epidermis to the dermis [20]. Based on the electron microscopic appearance, the zone is the collective name for four distinct layers. Put simply, hemidesmosomes from the basal keratinocytes anchor keratinocytes to the basal lamina (basal lucida and densa) which is in turn bound to the anchoring plaques in the superficial papillary dermis by anchoring fibrils composed of type VII collagen [1].
5. Functions
5.1. Barrier function
5.1.1. Water homeostasis
The importance of the skin as a barrier is illustrated by the mortality associated with large surface area burns, where increased transepidermal water loss culminates in dehydration, renal failure and shock. Whereas other land-living animals such as insects have adapted by possessing a rigid cuticle coated with hydrocarbons, mammals have evolved a unique tough, flexible stratum corneum [21].
A simplistic view of the structure of the stratum corneum is the Brick and Mortar hypothesis, which depicts 10–20 layers of brick-like corneocytes, held together by corneodesmosomes (rivets), embedded in a hydrophobic extracellular lipid matrix (mortar), and enclosed in a protein envelope (Fig. 5) [22].

Eighty percent of the corneocytes is keratin, whose filaments are aligned into disulfide cross-linked macrofibres under the influence of filaggrin [23]. This protein is secreted as a precursor pro-filaggrin, released from the keratohyalin granules of the granular layer. The tortuous pathway between the stacked corneocytes is filled with “mortar” lipids, which are secreted into the intercellular space from the lamellar body secretory system of the granular layer as an intercellular lipid membrane bilayer consisting of three class of lipids: ceramides, cholesterol and free fatty acids in an equimolar ratio (Fig. 5) [24]. Using a water-soluble tracer injected into the dermis, an upward movement of water has been shown to occur between the epidermal cells, but efflux is blocked at the stratum granulosum/stratum corneum interface. From here on, water is lost in vapor form, but it is also trapped within the corneocytes, plasticizing the keratin. Thus, the lipids seal in the moisture but enables transepidermal water loss [25].
The cornified cell envelope is formed from the cross-linking of proteins loricrin and involucrin, also produced from the keratohyalin granules, following the action of calcium-dependent epidermal transglutaminase [26].
The stratum corneum also contains a battery of lipolytic and proteolytic enzymes involved with processing of probarrier lipids, mediating desquamation of corneocytes, and degradation of profilaggrin to filaggrin, which is further broken down into a blend of amino acids collectively called the NMF, or Natural Moisturizing Factor, which plasticizes keratin [27].
5.2. Immunological barriers
5.2.1. Innate immunity
Skin is an active immunological organ, and dysfunctional innate defenses have serious clinical implications. Products of the stratum corneum, including free fatty acids, polar lipids, and glycosphingolipids accumulate in the intercellular spaces and horny layer, exhibiting antimicrobial properties, and functioning as a first line of defense. Antimicrobial peptides (AMPs) exhibit potent and targeted resistance against a wide spectrum of common pathogens [28]. When this barrier is breached, second lines of protection are provided by inflammatory cascades in the subepithelial tissue.
Approximately sixteen AMPs have been shown to be expressed in the skin (Table 1). The main antimicrobial families are known as cathelicidins and defensins [29,30]. Defensins are classified as human beta and alpha defensins, based on their size and pattern of disulfide bonding, and have been shown to be localized to the basal layer of keratinocytes, in the dendritic cells of the stratum spinosum, in the dermal glandular structures and in hair follicles [31]. Human α-defensin 1 and 3 (DEFA1 and DEFA3) are secreted by neutrophils and play a major role in phagocyte-mediated host defense against viral hemorrhagic septicemia rhabdovirus, adenoviruses, fungi, and exhibits potent antimicrobial activity against gram-negative and gram-positive bacteria, including Burkholderia cepacia [32,33]. The antimicrobial mechanism(s) of action of defensins comes from their ability to penetrate antigenic membranes, where they form channels or “pores,” ultimately destroying the bacterium by altering membrane conductance and affecting intracellular function, with a consequent lack of antigenicity and chemical and biological resistance.