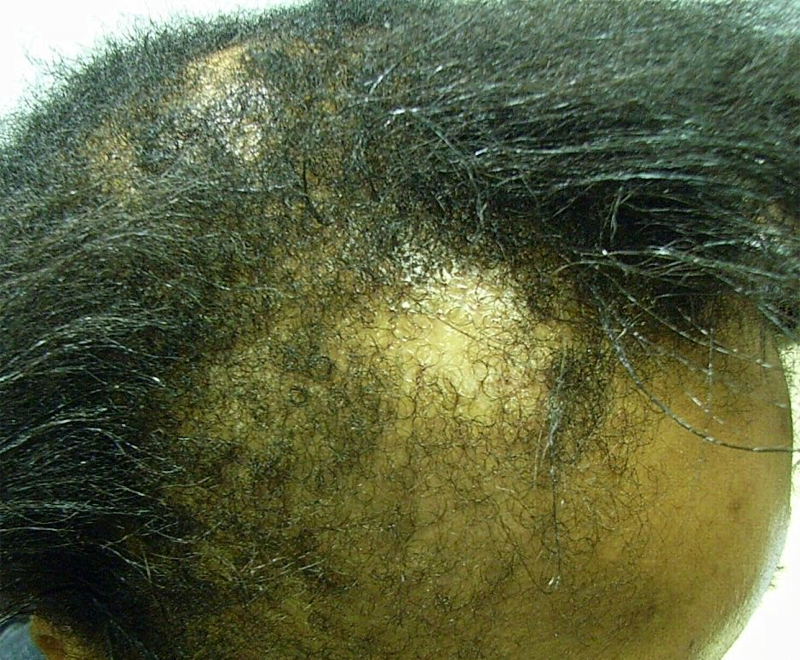
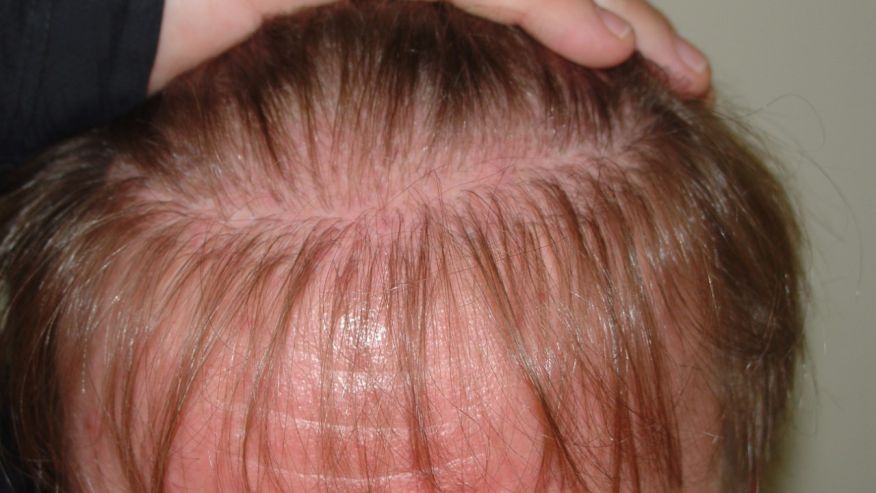
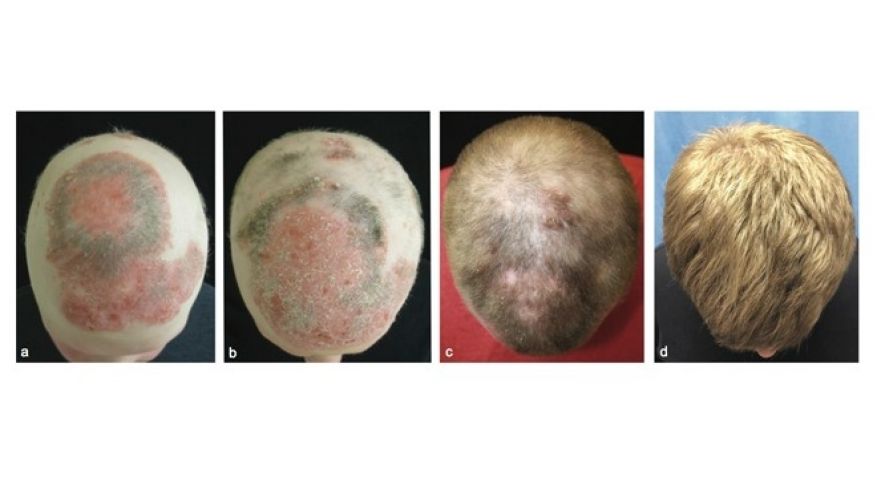
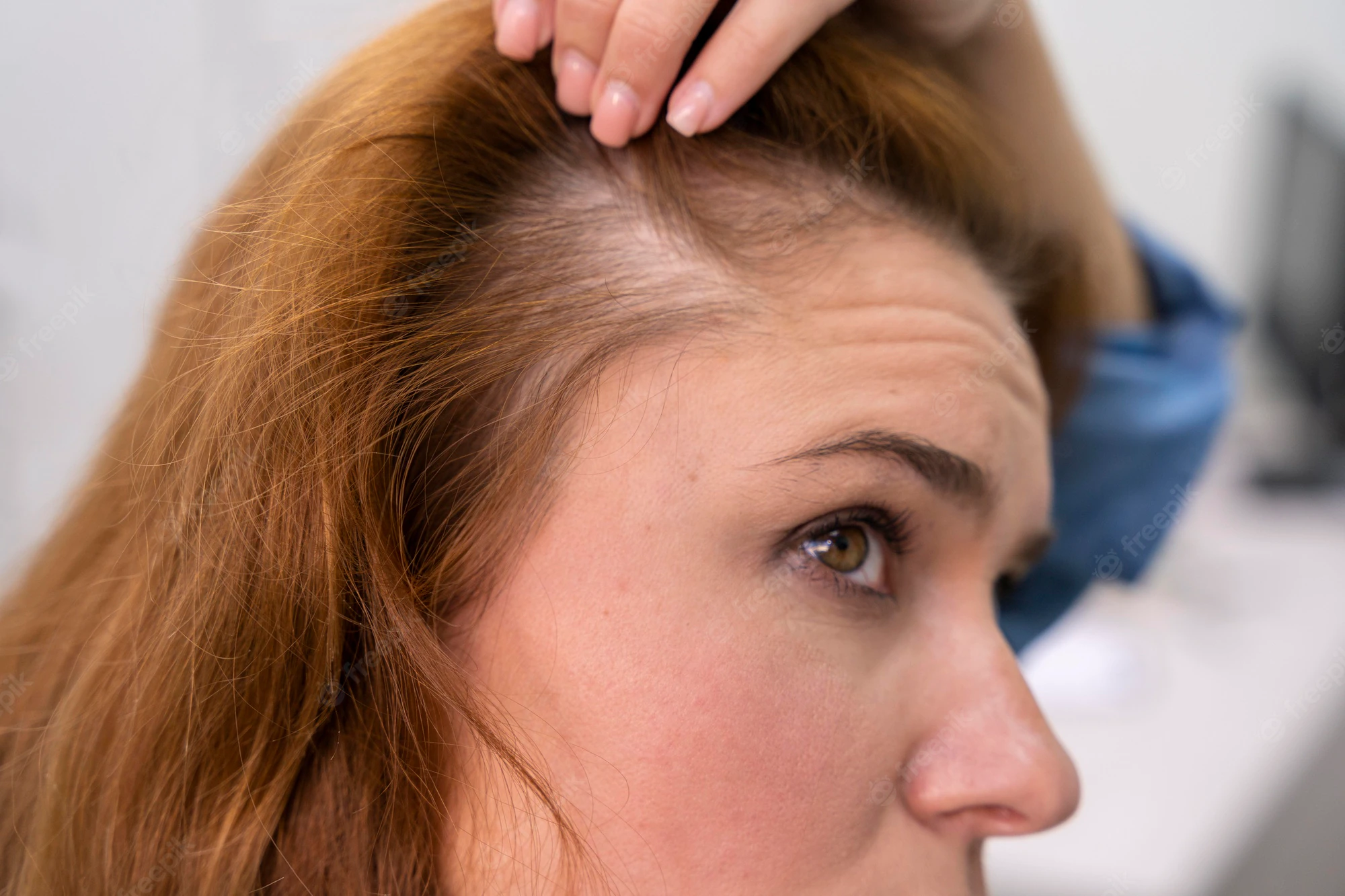
Lilly Korea is to conduct a clinical trial on rheumatoid arthritis drug Olumiant (ingredient: baricitinib) to check whether it will work for alopecia areata, also known as spot baldness.
The Ministry of Food and Drug Safety on Monday approved the drugmaker to go ahead with a phase-2/3 study on barcitinib (LY3009104).
 |
| Lilly Korea’s rheumatoid arthritis drug Olumiant |
The study will be a multicenter, randomized, double-blind, placebo-controlled, operationally seamless, adaptive phase-2/3 trial to evaluate the efficacy and safety of baricitinib in adult patients with severe or very severe alopecia areata (BRAVE-AA1).
The global study will also take place in Korea. Participating hospitals include Catholic University of Korea St. Paul’s Hospital, Gangdong Kyung Hee University Hospital, Konkuk University Hospital, Kyungpook National University Hospital, Pusan National University Hospital, Seoul National University Bundang Hospital, Seoul National University Hospital, Inha University Hospital, Chonbuk National University Hospital, Chung-Ang University Hospital, and Chungnam National University Hospital.
Baricitinib is a Janus kinase (JAK) 1/2 inhibitor that suppresses the increase of inflammatory cytokine by blocking intercellular communication paths of JAK.
The agent won approval under the brand name of Olumiant in the U.S. and Korea. Pfizer’s Xeljanz is leading the oral rheumatoid arthritis drug market. Unlike Xeljanz’ dosage of twice a day, Olumiant can be taken once daily. With better user convenience, Olumiant is rapidly broadening its market share.
Lilly is also testing baricitinib’s efficacy in patients with atopic dermatitis around the world. With the study on barticitinib’s effect on spot baldness, the pharmaceutical firm is seeking to expand the treatment’s indication.
[“source=koreabiomed”]