Stem cells switch off and on, sometimes dividing to produce progeny cells and sometimes resting. But scientists don’t fully understand what causes the cells to toggle between active and quiet states.
New research in Elaine Fuchs’ Laboratory of Mammalian Cell Biology and Development focused on stem cells in the hair follicle to determine what switches them on. The researchers found cells produced by the stem cells, progeny known at Transit-Amplifying Cells or TACs, emit a signal that tells quiet hair follicle stem cells to become active.
“Many types of mammalian stem cells produce TACs, which act as an intermediate between the stem cells and their final product: fully differentiated cells in blood, skin and elsewhere,” says Ya-Chieh Hsu, who conducted the research while as a postdoc in the lab and will soon move to Harvard University. “In the past, TACs were seen as a population of cells that sat by passively cranking out tissues. No one expected them to play a regulatory role.”
Hsu and Fuchs went a step further to identify the signal sent out by the TACs. They pinpointed a cell-division promoting protein called Sonic Hedgehog, which plays a role in the embryonic development of the brain, eyes and limbs.
Stem cells are medically valuable because they have the potential to produce a number of specialized cells suitable for specific roles. Stem cells’ production of these differentiated cells is crucial to normal maintenance, growth and repair. Many tissues have two populations of stem cells: one that divides rarely, known as the quiescent stem cells, and another that is more prone to proliferate, known as primed stem cells. Regardless of their proliferation frequency, most stem cells in humans do not directly produce differentiated progeny cells; instead, they give rise to an intermediate proliferating population, the TACs.
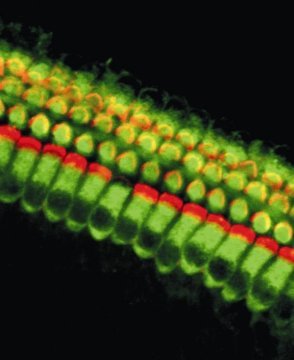
The hair follicle, the tiny organ that produces a hair, forms a narrow cavity down into the skin. It cycles between rounds of growth, destruction and rest. When entering the growth phase, the primed stem cell population is always the first to divide and generates the TACs clustered lower down in the hair follicle. Primed stem cell proliferation sets the stage for the next round of hair growth, a process which ensures hairs are replaced as they are lost over time. Proliferating TACs produce the hair shaft, as well as all the cells surrounding the hair underneath the skin, which make up the follicle itself.
At the outset, Hsu and Fuchs suspected a role for both the TACs and for Sonic Hedgehog in hair regeneration.
“We noticed that the primed stem cell population gets activated early and makes the TACs, while the quiescent stem cell population only becomes activated once TACs are generated. This correlation prompted us to look for a signal that is made by the TACs. Sonic Hedgehog is that signal, as we went on to demonstrate,” explained Fuchs.
In experiments described this week in Cell, Hsu disabled TACs’ ability to produce the Sonic Hedgehog protein by knocking out the gene responsible in the hair follicles of adult mice. As a result, the proliferation of hair follicle stem cells and their TACs are both compromised. They further showed that it is the quiescent stem cell population which requires Sonic Hedgehog directly for proliferation.
Surprisingly, when Hsu blocked the ability of the quiescent stem cells to respond to Sonic Hedgehog, hair growth proceeded, but follicles were shorter, and with each round of hair cycling, the quiescent and primed stem cell populations were diminished, until hair regeneration failed altogether. These features are remarkably similar to what happens in male pattern baldness, according to the researchers. Although the root of this disorder may be further upstream than Sonic Hedgehog, this study provides new insights into the manifestations of hair loss, which in the long run will be necessary to develop new therapeutics, they say.
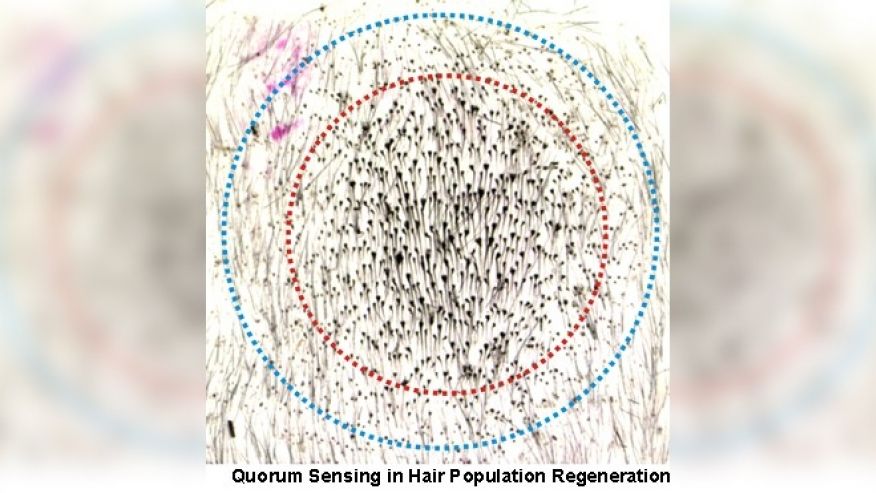
TACs are a step in many different stem cell lineages, and the researchers posit that although the precise signal may differ, TACs may be functioning similarly in other tissues such as our blood and intestine. “In most adult tissues,” Fuchs says, “our stem cells must be able to quickly respond to injury. By exploiting the TACs as rheostats, the process can be tightly regulated to ensure that just the right amount of new tissue is generated to repair the wound. Thus, TACs can no longer be viewed as a passive, intermediate in a stem cell lineage but rather as a key signaling center that orchestrates tissue regeneration.”
[Source:- Science Daily]