| Abstract |
Female pattern hair loss (FPHL) is nonscarring progressive thinning of hair with gradual decrease in the number of hair, especially in the frontal, central, and parietal scalp, due to a process known as follicular miniaturization. The etiopathogenesis of FPHL is complex with multiple factors such as genetics, inflammation, hormones, and environment playing role in it. It usually manifests as slowly progressive hair thinning, mainly over the vertex and upper parietal scalp, the frontal hairline is often spared and the miniaturization is also not as severe as in men. A thorough history, clinical examination, hair loss evaluation tests, dermoscopy, and scalp biopsy can help in establishing the diagnosis. Various biochemical tests may be needed in patients with hyperandrogenism. The treatment includes medical and surgical modalities. Topical minoxidil is still considered the first line of treatment. Along with medical therapy, cosmetic camouflage may also be needed in some cases.
| Introduction |  |
Female pattern hair loss (FPHL) is nonscarring progressive thinning of hair with gradual decrease in the number of hair, especially in the frontal, central, and parietal scalp, the loss of terminal hairs in affected areas is usually incomplete and the frontal hairline is often spared. It is due to a progressive decrease in the ratio of the terminal to vellus hair—a process known as follicular miniaturization.[1] The term female pattern baldness was used for diffuse alopecia in women since it was thought to be a variant of androgenetic alopecia in women.[2] The role of androgens in the development of female pattern baldness has not been fully demonstrated, hence, the term “FPHL.”[3]
Etiopathogenesis
The etiopathogenesis of FPHL is complex with genetic, hormonal, and environmental factors playing a key role in it [Figure 1].[4]
 |
In FPHL, there is a reduction in the duration of the anagen phase along with the miniaturization of the dermal papilla. Miniaturization is the progressive transformation of terminal hair follicles (large, thick, pigmented) to vellus-like hair follicles (short, thin, nonpigmented) and is the hallmark feature in FPHL.[5] These vellus-like hair follicles have a shortened hair cycle because of the reduction in the anagen phase, which leads to the production of fine and short hair shafts. Thus, premature termination of the anagen phase is the key event in the development of FPHL.[6] In addition, there is a delay between the end of the telogen phase and the beginning of the new anagen phase—the so-called kenogen phase, in which the hair follicle remains empty.[7]
The diameter of the hair follicle is determined by the size of the dermal papilla and thus miniaturization process is due to a decrease in the volume of the papilla which takes place somewhere between the catagen and the formation of new hair. The factors that lead to miniaturization are not fully known but a possible mechanism is a decrease in the number of papilla cells due to apoptosis.[2] The follicular regression in the catagen phase is the outcome of the diffuse apoptosis of follicular keratinocytes.[7],[8] Apoptosis occurs as a result of an imbalance between various growth factors and cytokines that promote apoptosis such as fibroblast growth factor 5, interleukin 1 alpha, prostaglandin (PG) D2, transforming growth factor-beta 1, tumor necrosis factor-alpha 1, and those growth factors and cytokines which maintain the anagen phase such as basic fibroblast growth factor, fibroblast growth factor 7, hepatocyte growth factor, insulin-like growth factor 1, PG-E2, vascular endothelial growth factor and Wnt signaling pathway [Figure 2]a.[2]
 |
- Hormones: The role of androgens in the development of FPHL is not yet clear. In fact, FPHL occurs in some females with normal levels of circulating androgens.[6] The role of enzyme 5α- reductase, which converts testosterone to dihydrotestosterone (DHT) is not clear.[9] DHT has five times greater affinity for the androgen receptor (AR) than testosterone. The androgen linked to the AR leads to the transcription of the genes responsible for its biological action on the target cells. In addition, DHT may interfere with the follicular cycle by interacting with the Wnt signaling pathway which induces dermal papilla cells to maintain the anagen phase. Aromatase is an enzyme that converts androstenedione to estrone and testosterone to estradiol, exerting an antiandrogenic action, hence, may have a protective role in FPHL [2]
Thyroxin and prolactin interfere with androgen metabolism, hence, they may contribute to the clinical picture of female pattern alopecia.[10] However, a study showed that only high doses of prolactin have an inhibiting effect on human hair follicles while the role of moderately elevated prolactin can be neglected.[11] FPHL has been associated with insulin resistance and other manifestations of metabolic syndrome that may be attributed to vascular impairment due to hyperglycemia which could damage hair follicles and contribute to hair loss.[12]
- Microinflammation: In baldness, a mild to moderate lymphohistiocytic inflammatory infiltrate in the peri-infundibular region is present along with miniaturization. The term “microinflammation” has been used to differentiate it from the inflammation that occurs in scarring alopecia. The inflammatory process occurring in the upper part of the follicle suggests that the causal factor may affect this region. Various factors such as ultraviolet radiation, environmental pollutants, inhabitants of the skin microbiota and follicle (such as Propionibacterium sp.; Staphylococcus sp.; Malassezia sp.) may be involved in the induction of the microinflamation process [13]
- Genetics: In women with normal androgen levels, a genetic predisposition may be involved. Patients with FPHL often report family members affected by the disease (40–54%), especially in cases with early clinical presentation (<40 years).[14] The fact that FPHL manifests with varying degrees of intensity and has its onset at different ages suggest a polygenic pattern with incomplete penetrance.[2] The strongest evidence of genetic involvement in the development of baldness comes from studies involving the AR gene in men. Single nucleotide polymorphism in the first exon, known as STUL was associated with baldness. However, the relationship between the presence of the STUL restriction fragment and FPHL has not been established.[2]
In women with FPHL (especially in younger women), nonfunctioning single nucleotide polymorphism (rs4646) was identified in the gene that encodes aromatase (CYP19A1). A meta-analysis of seven genome-wide association studies identified six susceptibility loci associated with MPA: 1p36.22, 2q37.3, 7p21.1, 7q11.22, 17q21.31, and 18q21.1.[15] However, another study found no association between these loci and FPHL which suggest that MPA and FPHL have different etiopathogenic factors.[2]
- Environment: Hair loss in females is polygenic and multifactorial with the additional influence of environmental factors.[6] Several environmental factors possibly related to FPHL are psychological stress, hypertension, diabetes mellitus, smoking, multiple marriages, lack of photoprotection, higher income, and little physical activity [Figure 2]b.[16]
Clinical features
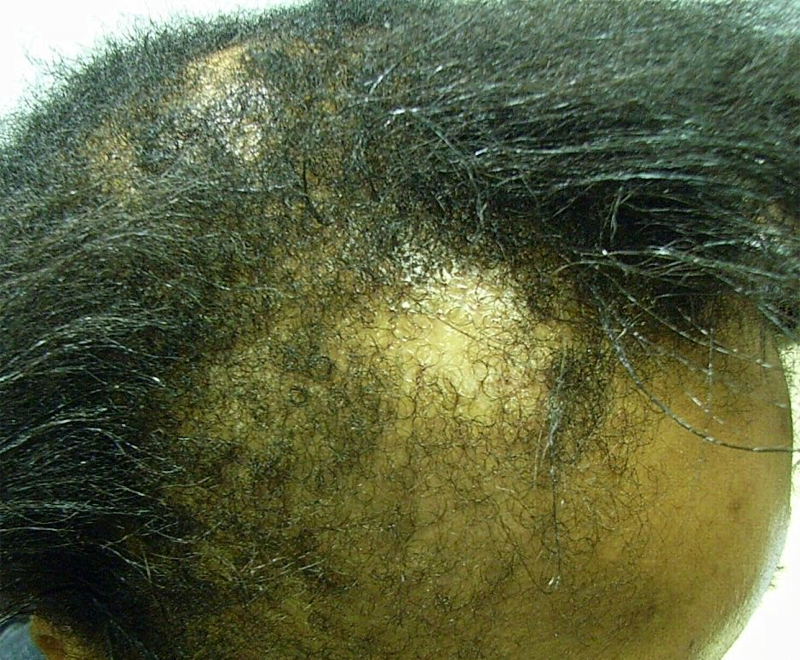
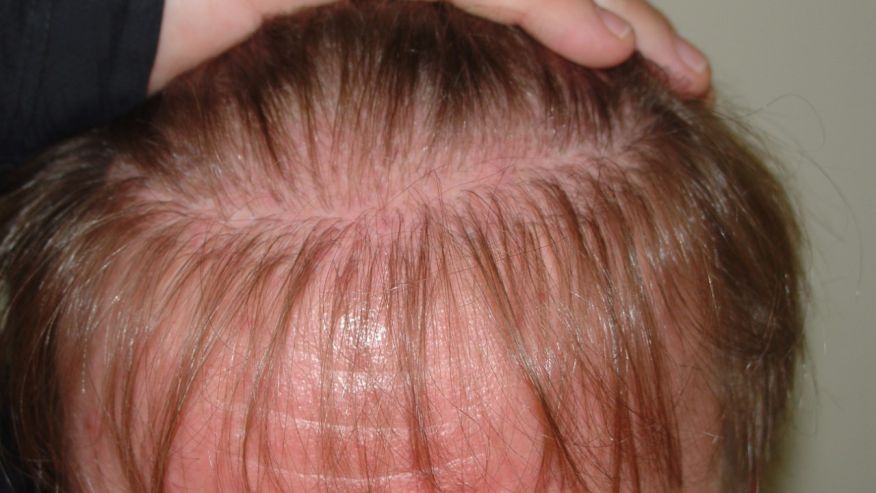
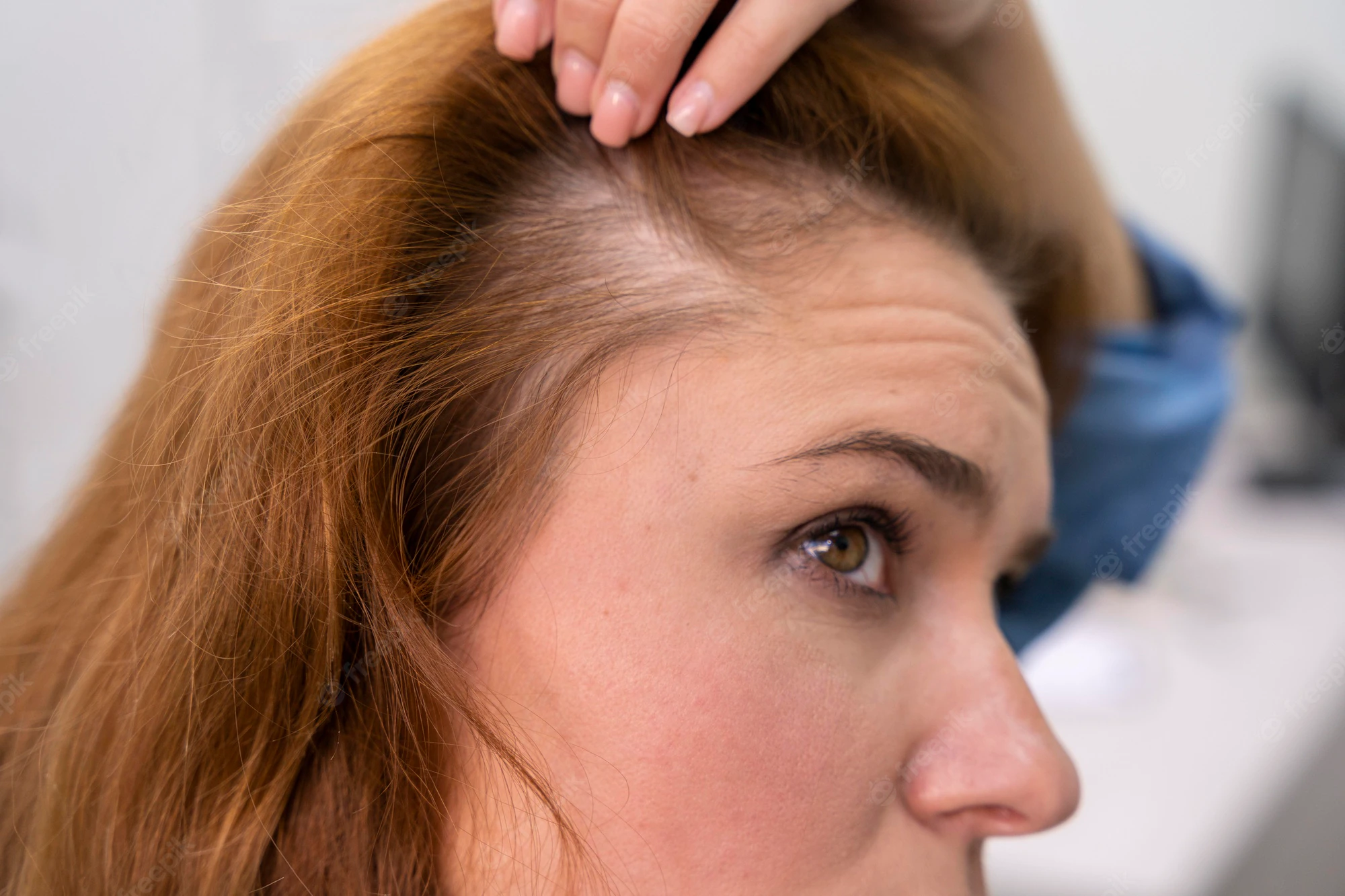
FPHL usually manifests as slowly progressive hair thinning, mainly over the vertex and upper parietal scalp, that may or may not be associated with increased shedding. Unlike in men, the frontal hairline is often spared and miniaturization is also not as severe [Figure 3]. However, in some women, the hair thinning is more diffuse, involving the parietal and occipital areas of the scalp with a pattern of diffuse alopecia.[17] Commonly, FPHL may have three different patterns:
 |
- Diffuse thinning of the crown region with the preservation of the frontal hairline with two scales being used to describe this pattern: the commonly used 3-point Ludwig scale and the 5 pointer Sinclair scale.
- Thinning and widening of the central part of the scalp with breach of frontal hairline, described by Olsen scale—like the Christmas tree pattern. In this, in addition to the diffuse thinning process, there is an accentuation in the central line, opening up into a triangle at the anterior hairline.
- Thinning with bitemporal recession: Hamilton-Norwood scale.
All these patterns spare the occipital region and may be attributed to the mesodermal origin of the dermis of the occipital region in contrast to the neural crest origin of the dermis of frontal/parietal scalp.[18]
Classification systems
- Ludwig classification: Given in 1977, the classification consists of three grades of hair loss, which include:
Grade 1: Perceptible thinning of the hair on the crown, limited in the front by a line situated 1–3 cm behind the frontal hairline,
Grade 2: Pronounced rarefaction of the hair on the crown, within the area seen in grade 1 &
Grade 3: Full baldness (total denudation) within the area seen in grades 1 and 2.[19]
- Sinclair’s Classification: given by Sinclair et al.[20]
- Olsen’s classification: Other than diffuse thinning, there is also a frontal accentuation opening toward the anterior hair implantation line, creating a “Christmas tree” pattern.[21],[22]
- BASP Classification: The basic type (BA) is defined by the shape of the anterior hair implantation line. There are four types and they are designated by letters. Type L is a linear pattern in which there is no hair loss in the frontal hair implantation line. In type M, the bilateral frontotemporal recess is more prominent than the central recess, having a shape of letter M. In Type C, the central recess is more prominent than the frontotemporal recess. Types M and C are subdivided into four groups, according to the intensity. Type U, the frontal line is behind the vertex. It has the shape of a horseshoe or of the letter U. Type U is subdivided into three groups, according to the position of the hair implantation line between the vertex and the occipital protuberance. The specific type (SP) represents the capillary density in certain areas. Type F (frontal) has decreased hair density across the top area of the scalp, except the anterior line. Type V is the rarefaction in the vertex region. SPs are subdivided into three groups, according to the intensity. When the patient presents with both types (F and V), both types should be described. The final type is decided by the combination of the assigned basic and SPs.[23]
Severity grading scale for female pattern hair loss (FPHL)
Kaneko et al. have devised a grading system for early FPHL with five levels focusing on the changes revealed by the surface reflected light of flash generated on global photographs (GP).[24] FPHL-SI (FPHL severity index) includes four evaluation items—the amount of hair shedding, midline hair density, hair diameter variation, and difference in the proportion of single hairs per hair follicle unit between the frontal and occipital scalp. Amount of hair shedding is graded as less than 200 hairs shed per day, negative pull test as 0 point, and positive pull test as 2 points. Midline hair density is assessed according to Sinclair’s midline hair density scale. In trichoscopy images, a hair diameter variation of <20% is considered point 0, and that of 20% or more is considered 5 points. No difference in the amount of hair included in the follicular unit between the forehead and in the occipital area is evaluated as point 0, a difference of more than 25% is assessed as point 1, and difference of more than 50% is 2 points.[25]
Diagnosis
A detailed medical history should be taken and physical examination should be performed on every patient of FPHL. This will help in the diagnosis and treatment of other concurrent medical conditions.
- Pull test: When the test is positive in FPHL, it is restricted to the areas affected by the disease. The presence of telogen hairs smaller than 3 cm represents the telogen phase of the miniaturized follicles and is strongly suggestive of the diagnosis of FPHL [26]
- Standardized wash test: This test differentiates between TE and FPHL.[9] In FPHL or AGA, the loss is less than 100 hairs with 10% or more being telogen vellus hairs
- Modified wash test: Also called as androgenetic/telogen effluvium wash test, it distinguishes between TE and FPHL. The hairs are counted and divided into three groups by hair length, i) long hair >5 cm; ii) intermediate-length hair (>3 to <5 cm); iii) short vellus hairs (<3 cm). Hairs <3 cm are considered as telogen vellus hairs. The final results are calculated as a total number of telogen hairs and the percentage of telogen vellus hairs [9]
- Trichogram: The trichogram is a semi-invasive microscopic method for hair root and cycle evaluation. By this method, hair follicles are quantified in different growth phases of hair cycle and anagen/telogen ratio is determined. Trichogram has been found pathological in 62% of those with early FPHL and 84.2% in advanced FPHL.[27] The unit area trichogram can also be used in which a fixed area is sampled
- Phototrichogram: It is a technique that makes it possible to determine the density and the percentage of anagen and telogen hair in a selected area on the scalp by taking magnified images of the scalp using a light microscope or a dermoscope [28]
- Trichoscopy: Dermoscopy can contribute to the diagnosis of FPHL, especially in the early stages of the disease. The main dermoscopic finding is the diversity in the thickness of the hairs with an increased number of miniaturized hairs, especially in the frontoparietal region [Figure 4]. Hair diameter density >20% is diagnostic for FPHL. Short vellus hair (<0.03 mm) is a sign of severe miniaturization and their presence on the frontal scalp is a very useful clue for FPHL. A decrease in the number of hairs per follicular unit is another important element.[29] The peripilar sign, a light brown area, slightly atrophic around the follicle, usually occurs in the early stages of FPHL which correlates with the inflammatory infiltrate. It can be brown sign, which is seen in early grades of FPHL and white peripilar sign, seen in later grades.[30] Yellow dots may be seen in more advanced cases, probably as a result of sebum and keratin accumulation in dilated follicles. With increased thinning of the hairs, there is greater penetration of ultraviolet radiation into the scalp and changes typical of photoaging, such as the honeycomb pigmented network, may occur. These signs, when evaluated together, allow the early diagnosis of FPHL. In 2009, Rakowska standardized the criteria for the diagnosis of FPHL based on dermoscopic findings [Figure 5][31]
- TrichoScan: TrichosScan ® is a system that combines epiluminescence microscopy and automated digital image analysis. It allows the estimation of the number and density of hairs, of the percentage of the terminal and vellus hairs, and by mathematical approximation, of the percentage of hairs in the anagen and telogen phases
- Scalp biopsy: It is the most reliable test to differentiate FPHL from CTE by evaluation of terminal:vellus hair ratio. A ratio of <4:1 is diagnostic of FPHL and a ratio of >8:1 is diagnostic of CTE [19]
- Laboratory investigations: The European Consensus held in 2011 recommends the free androgen index (FAI) and prolactin levels as screening tests. The FAI is the relationship between total testosterone and sex hormone-binding globulin (SHBG) [total testosterone (nmol/L)/SHBG (nmol/L) × 100]. FAI of 5 or greater is indicative of polycystic ovary syndrome. The use of hormonal contraceptives causes alterations in SHBG levels. Therefore, laboratory tests should only be carried out after a hormonal contraceptive pause of at least 2 months. Thyroid function should be evaluated as thyroid dysfunctions may contribute to the effluvium associated with FPHL [Figure 6].[32]
 |
 |
 |
Treatment
Treatment options for FPHL can be classified as “topical” or “systemic.”
- Topical therapy:
- Minoxidil: It is a piperidinopyrimidine derivative and potent vasodilator. It is a pro-drug that is converted to its active form, minoxidil sulfate, by sulfotransferase enzymes in the outer root sheath of hair. Minoxidil 2% twice daily was approved by the FDA in 1991 for FPHL and a 5% minoxidil foam once daily was approved in 2014.[33] For FPHL, a randomized trial showed that the 5% minoxidil foam once daily was similar in efficacy to the 2% minoxidil solution twice daily for the treatment of female AGA.[34] Minoxidil is a potassium channel opener and stimulates hair growth by increasing the anagen phase of the hair cycle. It enhances angiogenesis around the follicle but the exact mechanisms are currently unknown.[35],[36] The topical minoxidil 2% solution should be applied only to the affected area of the scalp at 1 mL twice daily (once daily for the minoxidil 5% foam) for a minimum period of 12 months before determining the efficacy. Side effects include allergic or irritant contact dermatitis mostly due to vehicle propylene glycol, hypertrichosis of the forehead or face [17]
- Prostaglandin analogs: Among the prostaglandins, the PG-F2 analogs latanoprost and bimatoprost are known to stimulate hair growth by prolonging the anagen phase.[37] In a study conducted in men with AGA, 0.1% latanoprost significantly increased hair density and pigmentation at 24 weeks compared with baseline and compared with the placebo-treated site. Other studies have revealed that an increased PG-D2 level is correlated with the miniaturization of hair follicles and its topical application inhibited hair growth.[38] Recent research studies focus on drugs that are able to block the PG-D2 receptor (GPR44). Setipiprant (KITH-105) is an orally administered GPR44 receptor inhibitor which may be helpful [39]
- Ketoconazole: It has an anti-inflammatory property and also acts as the androgen-receptor antagonist which may explain the efficacy of topical ketoconazole in AGA [40],[41]
- Melatonin: Melatonin is known to have strong anti-oxidant properties and the ability to actively capture free radicals.[42] Melatonin has been found to modulate hair growth, pigmentation, and molting in many species including humans.[43] The topical application of the melatonin 0.1% solution was shown to significantly increase anagen hair in male and female AGA with good compliance in a controlled study [44]
- Platelet-rich plasma: it is an autologous concentration of human platelets contained in a small volume of plasma and is composed of several different growth factors—platelet-derived growth factor, transforming growth factor-alpha, vascular endothelial growth factor, insulin-like growth factor 1, epidermal growth factor, basic fibroblast growth factor, transforming growth factor-beta1, and platelet-activating factor that are released through degranulation. Various studies have reported improvement in regrowth rates after five local treatments of 3 mL of platelet-rich plasma (PRP) at 2–3-week intervals and histology examinations showed thickened epithelium, the proliferation of collagen fibers and fibroblasts, and increased vessels around follicles [45]
- Microneedling: It is a minimally invasive dermatologic procedure in which fine needles are rolled over the skin to puncture the stratum corneum. The physical trauma from needle penetration in microneedling induces a wound healing cascade with minimal damage to the epidermis stimulating collagen formation, neovascularization, and growth factor production in the treated areas. Microneedling enhances drug delivery in the treatment of AGA and FPHL and has been successfully paired with other hair growth-promoting therapies such as minoxidil, PRP, and topical steroidal medications [46]
- Light treatments: Low-level light therapy (LLLT) is a relatively new technique in the treatment of AGA. The mechanism is not completely understood but the cellular respiratory chain of mitochondria probably absorbs the light energy, which results in increased electron transport and the promotion of cellular signaling which, in turn, causes hair regrowth.[47] Several LLLT devices available for the treatment of alopecia include a comb, hood, and helmet. However, the efficacy of LLLT devices remains unclear due to the lack of proper studies. A 1550 nm fractional erbium-glass laser irradiation may be an effective and safe treatment option for FPHL [48]
Other topical treatment options are summarized in [Figure 7].[5]
 |
- Systemic treatment:
- Finasteride: It works by inhibiting the 5α-reductase II enzyme which catalyzes the conversion of testosterone to the much more active chemical 5-DHT [Figure 8]. It is not FDA-approved for use in women and contraindicated in pregnant women and during lactation due to the risk of feminization of the male fetus. Large scale studies on its efficacy for FPHL are limited. Price et al. proved that finasteride 1 mg/day taken for 12 months in postmenopausal women with AGA was ineffective.[49] However, various case reports and series have demonstrated the efficacy of low dosage finasteride in some cases of FPHL in both pre- and postmenopausal women. Shum et al. found that finasteride 1.25 mg/day improves patterned hair loss in women with hyperandrogenism but not in those without hyperandrogenism.[50] Won YY et al. evaluated 112 patients of FPHL and found that 29.5% of the patients studied showed slight improvement, 65.2% showed significant improvement, whereas no change was recorded in 5.4%. Efficacy of administration of finasteride at a dose of 2.5 mg/day for patients with FPHL was demonstrated. It was also found that finasteride has a better effect on hair growth when patients had a lower Ludwig score and an older age at onset.[51] Another study by Oliveira-Soares et al. showed oral finasteride 5 mg/day to be a safe treatment of FPHL in premenopausal women with adverse effects such as headache, menstrual irregularities, dizziness, increased body hair growth, decrease in libido and mastalgia.[52] However, these side effects are frequent, mild, and disappear over time, resulting in the long-term treatment of FPHL. This may be due to some adaptative hormonal changes in the hypophysis-gonadal axis or in brain perception. Further studies are needed to determine optimal dosing and efficacy of finasteride in FPHL
- Dutasteride: it is a 5α-reductase type I and II inhibitor [Figure 8] that has not currently been approved in men and women for the treatment of hair loss. Dutasteride can reduce serum DHT levels by 90% and has been reported to treat FPHL successfully with no side effects at doses that range from 0.25 to 0.5 mg/day.[53] However, it should not be given to women of childbearing age unless they are using birth control measures due to potential feminizing effects on the male fetus or to patients with impaired liver function
- Cyproterone acetate: it inhibits gonadotropin-releasing hormones and blocks ARs [Figure 8]. The treatment doses that were used in various studies vary but one of the most effective doses appears to be 100 mg/day on days 5–15 of the menstrual cycle and supplemented by 50 μg Ethinyl estradiol on days 5–25. There is insufficient evidence to prove that oral hormonal treatment prevents progression or improves AGA in female patients. However, some studies show that oral cyproterone acetate may improve AGA in female patients with hyperandrogenism [35]
- Spironolactone: It is a potassium-sparing diuretic, the structural antagonist of aldosterone and acts as an androgen antagonist [Figure 8] by competitively blocking ARs as well as inhibiting ovarian androgen production. It is the most commonly used anti-androgen for the treatment of FPHL and hirsutism. The usual daily dose is 100–200 mg. The side effects of spironolactone include postural hypotension, electrolyte disturbances, menstrual irregularities, fatigue, urticaria, breast tenderness, and hematologic disturbances. Studies suggesting the role of spironolactone in FPHL are limited.[20] Drospirenone is a synthetic progestin that is an analog to spironolactone. It has been found useful in FPHL due to its anti-androgen activity [54]
- Flutamide: It is an oral anti-androgen that acts by competitively inhibiting the uptake of androgen and its nuclear binding in target tissues. It has been shown to be effective for the treatment of FPHL in hyperandrogenic women at a dose of 250 mg per day [54]
- Oral minoxidil: It is not often used in the treatment of AGA and FPHL, mainly because of its side-effects. Off-label use of oral minoxidil has been shown to improve hair density in treated patients but can cause postural hypotension, fluid retention, and hypertrichosis. Low-dose oral minoxidil 0.25 mg is usually well-tolerated in the majority of patients with FPHL [55]
- Nutritional supplementation: The role of oral supplementation with amino acids, biotin, zinc, and other micronutrients in hair loss of any origin is controversial. The effectiveness of supplements in FPHL is doubtful. Iron acts as a metabolic cofactor for ribonucleotide reductase, which is the rate-limiting enzyme for DNA synthesis of hair growth stems. Therefore, the depletion of iron results in the inhibition of proliferation of stem cells. Recent studies have shown that vitamin D receptor activation plays an important role in anagen initiation and vitamin D receptors regulate the expression of genes that are required for hair follicle cycling.[56] Other ingredients such as saw palmetto (Nutrafol) or marine protein complexes (Viviscal) may have anti-androgenic and anti-inflammatory properties but their efficacy alone as a monotherapy needs more investigation.
 |
- Surgical techniques:
- Hair transplantation: It involves the transfer of hair follicles from the occipital to the bald area and is an important option for patients over 25 years of age with FPHL who do not respond to other medical therapies when the hair loss has been stabilized.[57] Ideal candidates for hair transplantation are women with high hair density in the occipital area and extensive hair loss in the frontal scalp. Follicular unit hair transplantation is the surgical procedure in which follicular units of hair are dissected under a stereomicroscope and transplanted in the bald area. The most common problems that are encountered in hair transplantation in women are related to insufficiency in hair donor areas, the need for magnification to insert the grafts and temporary worsening of the condition. PRP can be used as an adjuvant in hair transplantation. The growth factors and plasma components can be injected directly into the scalp before the placement of the grafts or the hair grafts may be stored in PRP until transplanted.[57] Moreover, robotic systems can be used for great precision and fewer efforts
- Camouflaging: The hair micropigmentation technique is a cosmetic nonsurgical procedure that results in the simulation of the appearance of hair follicles.[58] Camouflaging products and other concealment techniques can mask areas of visible hair loss. For mild-moderate loss, hair fibers (keratin-made), masking lotions, topical shading, and scalp spray thickeners may be used. For moderate-severe loss, integration hairpiece (fabric- or skin-like material) or wig may be used. For localized or diffuse patches, hats, scarves, bandanas, and turbans may be used.[59]
- Mesotherapy with growth factors: It involves injections of growth factors such as insulin-like growth factor-1 (IGF-1), basic fibroblast growth factor (Bfgf), vascular endothelial growth factor (VEGF), multivitamins, and amino acids into dermis/subcutaneous tissue. Mesenchymal stem cells can be processed, isolated, and used during mesotherapy
- Bioengineered hair follicle stem cells: Bioengineered germ cells can be used to grow hair follicles that are natural and follow the same cell dynamics and physiology as the original hair [60]
- Dermal sheath cup cells: Dermal papillae-derived cells and peribulbar dermal sheath cup cells are capable of dermal papillae formation and hair follicle induction when transplanted [61]
To conclude, FPHL is an underdiagnosed hair disorder with significant psychosocial morbidity in females. Its management is extremely important—the conventional treatment showing a variable response, newer treatment approaches are under investigation and appear to be highly promising.
[“source=idoj”]